|
This sim focussed on a life threatening presentation of a patient with end-stage renal disease (ESRD). Thanks to Suzy Connor and Andy Connor for their help with this simulation and information for this blog. The simulated case: A man in his 60s who has haemodialysis on Monday-Wednesday-Friday, presented on a Monday morning (see Fig 1 here) with shortness of breath and fatigue. Investigations revealed pulmonary oedema with hyperkalaemia and anaemia. Their case was discussed with the renal consultant on-call and they were transferred for dialysis. Afterwards we reviewed management of a major AV fistula bleed. What did we think? In debrief we discussed: Pulmonary oedema: Think about what questions you would ask the patient in these circumstances. Important renal points are (n.b. the patient’s “dry weight” is the estimated number of kilograms they aim to weigh following a dialysis session):
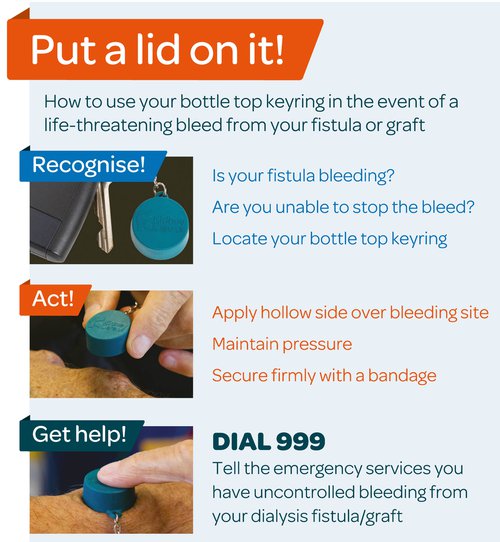
Other than this, it could be that their dry weight estimate is off e.g. they have lost muscle and fat mass without adjustment of their dry weight so now the body is made up of a higher proportion of water. If we are considering that a cardiac insult has caused the pulmonary oedema, the troponin result may be helpful if very high or much higher than previous. But obviously could be high from ESRD, so rely more on the ECG and history. Renal disease also puts the patient at risk of uraemic pericarditis which could cause the pulmonary oedema. In terms of treatments, loop diuretics are unlikely to help even if the patient produces urine as they often don't increase the output. A GTN infusion might help as a holding measure, but the definitive treatment is dialysis. Hyperkalaemia: Our hyperkalaemia protocol is found on the G:/ drive. It has a specific stream for fluid overloaded patients with the dextrose given at a higher concentration. Insulin and dextrose will work as a holding measure pending urgent dialysis. IV sodium bicarbonate may worsen fluid overload. Venepuncture and analgesia in ESRD: This patient has an arteriovenous fistula for dialysis and this is clearly very important for their survival. Avoid the fistula arm (which most likely will be on their non-dominant side) when taking blood, and go as distal as possible. Also avoid BP cuffs on the fistula side. Consider this too when taking blood from a patient who may require dialysis in the future and may therefore may need a AV fistula creating - go dominant side and as distal as feasible. If the attempt fails, escalate early rather than continue attempts. Pain relief can be tricky when renal function is poor. Generally the plan is to avoid opiates, although if necessary can use immediate-release low dose oxycodone. Avoid NSAIDs unless the patient has no urine output - in this case NSAIDs can actually be used as there is no renal function left to preserve. Who to escalate to? There is always at least one renal physician on-call 24/7: switchboard will advise who is first-on-call. We discussed that in this case it could be that ITU is needed due to the level of organ dysfunction. Bleeding AV fistula: For a dialysis patient, their fistula is their lifeline. Fistula problems can easily be life-threatening - have a really low threshold for admission. KidneyCareUK state: “Scabs at needling sites that are raised, enlarging bumps (aneurysms) along the access, shiny skin over the vascular access and/or loss of skin pigment over an aneurysm all require urgent review and, if left untreated, could potentially lead to a life-threatening bleed”.
To do:
There is a renal guidelines booklet on EDIS [ ] CAT tourniquet refresher video [ ] Consider the additional questions you might now ask a patient receiving dialysis when they present to the ED [ ] If you took part in the sim, you can use this blog as a starter to reflect on your own experience of it [ ] Blog by: James Keitley - ED sim fellow - on behalf of the ED sim team. --------------- For clinical decisions please refer directly to primary sources. This blog may not be updated. All images copyright- and attribution-free in the public domain or taken by the author.
0 Comments
This week we covered two scenarios, TCA overdose and a near-fatal asthma exacerbation. This blog will cover TCA overdose. Click the links to view previous blogs on paediatric and adult asthma. We’ve simulated TCA overdose earlier this year too but didn’t blog at the time, so here is a reflection of the scenario. Thanks to Annette and Laura for planning and running this sim. The simulated case: A teenage patient presents having taken an overdose of a tricyclic antidepressant, agitated and combative. What happened? The simulated patient was pre-alerted and team roles were assigned. Due to agitation, their initial assessment and treatment was challenging. They were given IM ketamine to facilitate assessment - this was “procedural sedation”, ideally with the preparation of gaining observations, IV access and ECG; not possible in the combative child/adolescent. Later they had several seizures. They were given IV sodium bicarbonate to counteract the cardiotoxic effect of amitriptyline. What did we think? In debrief we discussed: Danger of TCA overdose: The toxic dose is relatively low (see table on TOXBASE for different medications), so take it seriously. The concerning features are:
Anticholinergic syndrome could be recognised (tachycardia, mydriasis, dry mouth) and some TCAs can also contribute to serotonin syndrome. Bicarbonate:
We discussed how one of the central actions of TCAs on the heart is through blockade of sodium channels. The key management - sodium bicarbonate - may act through several mechanisms to interfere with this. TOXBASE has a helpful table of doses, but the bottom line if there are ECG changes, start the bicarbonate and titrate it according to the ECG. In cardiac arrest the adult dose is a rapid bolus of 100 mmol (i.e.100 mL 8.4%) sodium bicarbonate, or of 2-4 mL/kg of 4.2% if given peripherally in a child. If you only have a different concentration of bicarbonate, you can alter the amount to still give 100mmol (table on TOXBASE). 8.4% - 100mL 4.2% - 200mL 1.4% - 600mL 1.26% - 667mL It’s a really irritant drug to give, so ideally get a large vein and give a large flush following it, but it can be given into an intraosseous line as long as the line is flushed before administering other time critical medications. Note that in this case, amiodarone should be avoided as it can additionally extend the QT interval. It is also a situation where CPR may need to continue for several hours. Seizures: If not self-limiting, then IV benzodiazepines can be given. Seizures may be a sign that intubation and ventilation is required, although any drop in GCS below 13 should get you considering this or a rapid change in neurological status. Don’t wait for the seizure! Controlling the situation: This patient received IM ketamine. For pharmacological options, TOXBASE suggests oral, IV or IM benzodiazepines, IM haloperidol or ketamine. To do: Explore LITFL for ECGs in the case of tricyclic antidepressant overdose here [ ] Overview of toxicity here, or an EMJ review here [ ] If you took part in the sim, you can use this blog as a starter to reflect on your own experience of it [ ] Blog by: James Keitley and Annette Rickard on behalf of the ED sim team. --------------- For clinical decisions please refer directly to primary sources. This blog may not be updated. All images copyright- and attribution-free in the public domain or taken by the author. Recently we’ve run a few simulations where the participants cannot speak out loud and must focus on nonverbal communications, based on the EM3 plan. The original intention of the simulation is to hone skills that are helpful when our verbal communications are hampered by red PPE.
What did we think?
In debrief we discussed: The role of quiet: Silence in resus has its advantages. Distractions were minimal. It forced the closing of loops where people confirmed tasks had been received. And the team leader at the head of the bed in the amber zone was receptive to communications coming towards them. Reflect on any noisy patient encounters you have had and their effect on your stress, communication or decision-making. Perhaps noise was unavoidable in that situation, or perhaps it was greater than necessary. Part of the mitigation of this is systematic - how we design hospitals, monitors, room shapes, materials, doors, use of space and so on. But part we can influence, or at least be aware of when it is starting to impair processes so it can be highlighted. Two sides of the communication coin: Every time we receive verbal communication from a colleague it comes packaged with non-verbal cues e.g. posture, facial expression, eye contact or lack of, potentially written word and so on. These adjust the message received and can alter it drastically. They can reinforce what we are saying, or if incongruent they can confuse or completely change the message. So it is critical that our verbal and non-verbal sides are in-keeping with each other. When we wear red PPE we are reducing clarity of voice, availability of facial expressions, eye contact and more, and therefore putting ourselves at higher risk of being misunderstood. Clearly any hearing impairment of involved staff or patients is going to make this more challenging. As a baseline the understanding of a message is weighted towards the non-verbal cues, so now these are even more critical. Think about a time you have had a misunderstanding at work and which factors contributed to it. Perhaps it was a misunderstanding over email where we are stripped down to simply written word only, or on the phone with spoken word only. Perhaps HALT factors were involved and affected how the message was communicated. Perhaps there was confusion over who was to act on a spoken instruction because of a lack of direction or non-verbal back-up. Personally I can think of recent examples of all of these, they happen commonly. What can we do about these situations? We can ensure we specifically think about the power our non-verbal communication has.
These are just a couple of examples, have a think about specifics in your role. Awareness of others: It was pointed out during this sim that there is a risk of losing awareness of what others are doing within the team and focussing solely on your own task if there is no verbal communication. This highlights the fact that the ideal is to have both clear verbal and nonverbal aspects, including team mini-summaries to ensure everyone is on the same page and sharing the same mental model. To do: Think about a recent misunderstanding or a communication challenge and reflect on how you would change what happened next time [ ] NursingTimes article on nonverbal communication [ ] If you took part in the sim, you can use this blog as a starter to reflect on your own experience of it [ ] Blog by: James Keitley - ED sim fellow - on behalf of the ED sim team. --------------- For clinical decisions please refer directly to primary sources. This blog may not be updated. All images copyright- and attribution-free in the public domain or taken by the author. Continuing the gastro theme, here we worked through a case of decompensated liver disease. Thank you to Ash for being our sim patient and really committing to the cause! This week we also had a session of EMBites by Struan - look out for emails about upcoming sessions. They're short sharp reviews of key ED areas. The simulated case: A woman presented with abdominal pain and jaundice, having on a previous admission had several litres of ascitic fluid drained. What happened? The patient had tachycardia and slightly low oxygen saturations. The risk of spontaneous bacterial peritonitis (SBP) was recognised, so after an ascitic tap the patient was given IV antibiotics. Their results also showed acute kidney injury so they were given IV human albumin solution (HAS) as per the liver bundle. The CIWA protocol was started to plan for alcohol withdrawal and Pabrinex was given. What did we think? In debrief we discussed: Causes of decompensation: Have a think about what can trigger decompensation of liver disease. The liver bundle guide found on the G drive (search “decompensated”) can remind you of the common ones: alcohol, infection including SBP, and gastrointestinal bleeding. There is also constipation, portal vein thrombosis or development of hepatocellular cancer. Then think about what can cause the liver cirrhosis in the first place. Alcohol is an obvious one, but what else? The last few patients I have seen with decompensated liver disease have had non-alcoholic steatohepatitis (NAFLD). It’s possible to imagine and worth bearing in mind they might experience frustration at being questioned on alcohol at all steps of the healthcare system. There’s also the hepatitis viruses, primary biliary cholangitis, autoimmune hepatitis, Wilson’s disease and others. The patient in this case had jaundice and abdominal pain as their presenting complaints with a background of alcohol misuse. To prevent us narrowing the diagnostic frame too early, what might the wider differential diagnoses be? It could be pancreatitis, or alcohol-related pAF leading to mesenteric ischaemia. The abdominal pain with oxygen requirement could represent pneumonia, or acute coronary syndrome. The participants in the simulation obtained an ECG, chest x-ray, and blood tests that included a serum lipase. The admission mortality for decompensated liver disease is high at around 10-20%, and once decompensated, the risk of death within one year might be up to 80%. Bearing this in mind, it has also been reported that historically the care of these patients has not been optimal. The 2013 NCEPOD report on alcohol-related liver disease suggested that for the cases they investigated not enough was done to help people cut down on harmful alcohol use, fluid management was suboptimal, as was treatment of associated sepsis. So these patients can be really sick and need their symptoms and signs appreciated early. Don’t be afraid to raise concerns about them in a busy department. And don’t be afraid in the case of alcohol-related disease to ask them if they want to reduce their alcohol use - even if they have been coming in and out this might be the time they want to access help but haven’t brought it up. Brief interventions in the emergency department can help. And then there’s the alcohol liaison team on Salus or on a phone/bleep in working hours (I’ve put the numbers on the Induction app). Ash reminded us that these patients can often be in majors and risk ‘flying under the radar’. She reminded us of the importance of fully assessing the patient using the nursing assessment ‘as a tool to help us and not just a tick box’ and asking:
Use of human albumin:
The liver bundle advises use of human albumin solution (HAS) in two situations: 1.5g/kg if SBP has been diagnosed and either bilirubin is above 68 or creatinine above 88, or 1g/kg if the patient has stage 2-3 AKI (i.e. creatinine over double baseline). Patients with liver disease tend to be deficient in albumin and so have low oncotic pressure inside the blood vessels. This leads to vessels being “leaky” and losing fluid out into the peritoneal cavity (ascites) and other spaces. Therefore, giving them albumin solution is intended to increase oncotic pressure, keeping fluid within the blood vessels. In Derriford, 20% HAS comes in vials of 100mL, so 20g albumin per vial. This should be prescribed as separate entries on the fluid chart for each vial, with as many as takes you to the correct g/kg required. So a 90kg patient with stage 3 AKI (needing 1g/kg) requires 90g HAS, or 450mL of 20% solution. Ascitic tap: All patients with ascites presenting with decompensated liver disease require an ascitic tap. As per the BASL/BSG guidelines this does not need to wait for clotting blood results and can proceed regardless. It is worth noting here that even if INR is prolonged, patients with cirrhosis are at risk of blood clots and should have prophylactic enoxaparin unless actively bleeding or platelets <50. If it’s a procedure you would like to learn, seek out someone in the department or in the medical team who can teach it. For the test requesting, there’s an order set on ICM, under hepatology, that has the required tests that will print out the right stickers. In addition to this I’ve been taught to select blood cultures on top that you enter “ascitic fluid” in the comments of. So this produces 2 white top sterile universal containers, 1 lavender blood tube, and the blood cultures bottles. 1 white top and the blood cultures to microbiology, and 1 white top and 1 lavender tube to the biochemistry lab. It is needed to ring the microbiology technician on-call to alert them to the samples. An example guide to interpretation of results can be found here on GeekyMedics. Other features: Although this patient did not have these features, GI bleeding and hepatic encephalopathy are also important considerations. GI bleeding has been covered in a previous blog, and can also be reviewed on the liver bundle and GI bleeding protocols. Encephalopathy may present difficulties with providing care and with noticing pain/abnormal physiology. A precipitant cause should be looked for. Ensure lactulose (20-30mL QDS) is prescribed and consider other causes of delirium. To do: St Emlyn’s blog on treating decompensated liver disease in the ED [ ] This is the publicly available BASL/BSG liver bundle, but note there are differences in our local protocol especially in the AKI section. Our own can be found on the G:/ drive, search for “decompensated” [ ] If you took part in the sim or watched on the livestream, you can use this blog as a starter to reflect on your own experience of it [ ] Blog by: James Keitley - ED sim fellow - on behalf of the ED sim team. --------------- For clinical decisions please refer directly to primary sources. This blog may not be updated. All images copyright- and attribution-free in the public domain or taken by the author. Gastroenterology is the theme for March, so for this simulation we started off with an upper gastrointestinal bleed (UGIB). There’s a large spectrum with this presentation - this simulated patient was on the severe side. Thanks to Sally Pearson and Laura Jacobs for facilitating. The simulated case: A pre-alert was received for a man in his 50s who has been vomiting blood. He has had a recent binge of alcohol. Pre-hospitally he was identified as having a rapid pulse and low blood pressure. He had been cannulated and a 250mL bolus of fluid given. What happened? Before the patient’s arrival the team discussed roles and potential need for blood transfusion. The patient had blood taken and a second cannula inserted. After assessment the major haemorrhage protocol was initiated and blood transfusions initiated. The UGIB protocol was consulted and as this was a suspected variceal bleed the patient was given terlipressin and prophylactic antibiotics. What did we think? In debrief we discussed: Major haemorrhage protocol (MHP): We discussed that sometimes it’s appropriate to activate the MHP before the patient arrives based on what we know from the ATMIST. The MHP can be activated [edit 12/03/21: Sally has advised me that it is not through 2222, instead you must ring the blood bank directly] with a patient identifiable number. This is a resource-intensive process - really a specific person needs to raise the call and await callback from the blood bank, whilst another specific person is available to go and collect the blood products. Tell the EPIC. Remember we can also give O negative (or O positive in this case as male) blood which is stored within ED. Otherwise, blood can be crossmatched using the paper request forms that blood group samples are sent in. Remember to take the scanner to the patient and scan their wrist band to ensure no mislabelling occurs. There are logistical specifics that can be helpful here:
Electrolytes:
Low calcium is a problem in acute haemorrhage as it can further interfere with normal clotting. It can be triggered after giving the patient blood transfusions because of the use of citrate to impair clotting whilst the blood is being stored. Therefore calcium should also be administered. Since potassium is mainly intracellular, there is a risk that in stored blood some potassium moves outside of the cells, and especially in the case of any breakdown of the cells. Therefore massive transfusion comes with a risk of hyperkalaemia. If the patient also has a lactic acidosis from poor perfusion as a result of haemorrhage, then we know this can also increase serum potassium by pushing it out of cells into the blood in exchange for hydrogen ions. Further, hyperkalaemia can cause bradycardia - our patient is already having difficulty perfusing their organs so bradycardia here logically may add additional strain. Organising endoscopy: In-hours: inform medical registrar, endoscopist and gastroenterology registrar Out-of-hours: inform medical registrar and surgical registrar Liver and alcohol considerations: Clearly this patient will also need the liver bundle - see the guideline on the G drive - and the help of the alcohol liaison team. The UGIB guideline gives the dose of terlipressin to use if the bleed is suspected to be variceal. To do: Poiseuille’s law of flow applicable to IV cannulae [ ] Have you read the hospital massive transfusion policy? [ ] Two great guidelines to look at here: the UGIB one on EDIS, and the decompensated cirrhosis one on the G drive [ ] If you took part in the sim or watched on the livestream, you can use this blog as a starter to reflect on your own experience of it [ ] Blog by: James Keitley - ED sim fellow - on behalf of the ED sim team. --------------- For clinical decisions please refer directly to primary sources. This blog may not be updated. All images copyright- and attribution-free in the public domain or taken by the author. |
Categories
All
The Derrifoam BlogWelcome to the Derrifoam blog - interesting pictures, numbers, pitfalls and learning points from the last few weeks. Qualityish CPD made quick and easy..... Archives
October 2022
|